Recall the spectroscopic notation:
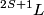 So, in this problem, the orbital angular momentum quantum number is 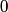 and the spin angular momentum quantum number is and the spin angular momentum quantum number is 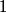 . Therefore, the total angular momentum quantum number can only have one possible value: . Therefore, the total angular momentum quantum number can only have one possible value: 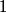 . Therefore, answer (B) is correct. . Therefore, answer (B) is correct. |